Sodium Hydroxide And Potassium Hydroxide Are Soluble In Water
Although it is considered to be. At room temperature sodium hydroxide is a white crystalline odorless solid that absorbs moisture from the air.
 Side By Side Comparison Potassium Hydroxide And Sodium Hydroxide Similarities Differences And Use Cases
Side By Side Comparison Potassium Hydroxide And Sodium Hydroxide Similarities Differences And Use Cases
Sodium hydroxide is highly soluble in water.
Sodium hydroxide and potassium hydroxide are soluble in water. Sodium hydroxide is an important industrial chemical where it is produced by the chloralkali process. About 121 g of KOH is soluble in 100 ml of water compared to 100 g of NaOH in 100 ml of water. Sodium hydroxide is very corrosive.
They are hygroscopic and deliquescent which means they absorb water from the environment and dissolve in it. Potassium hydroxide and sodium hydroxide are strong bases. In solid form they are both white powder or flakes.
Addition of only two equivalents of DMSO to potassium hydroxide formed in situ by addition of water to a solution of potassium naphthalene in THF was found to increase the rate of polymerization of ethylene oxide threefold. The residual content of 5-10 H2O is bound as a monohydrate. Potassium hydroxide and sodium hydroxide common inorganic compounds are used in various industries to produce their salts like carbonates and phosphates and a number of useful chemical compounds.
View chapter Purchase book. SODIUM HYDROXIDE AND POTASSIUM HYDROXIDE. The alkali metal hydroxides form white crystals that are hygroscopic and readily soluble in water generating large amounts of heat.
Sodium hydroxide Potassium hydroxide Sodium hydrate Potassium hydrate soda lye caustic potash CAS 1310-73-2 CAS 1310-58-3. So they are both highly exothermic. KOH is more soluble in water.
But the reaction with Sodium Hydroxide is slightly more exothermic which can make up for other more positive factors that Potassium Hydroxide possesses. 70 This increase cannot be due to an increase in the bulk dielectric constant of the medium since the amounts of added DMSO were small but solvation effects of the potassium. What is Soluble and Insoluble.
Both are hydroxides of alkali metals. It is a manufactured substance. Like all strong bases the reaction of both Potassium Hydroxide and Sodium Hydroxide with water is strongly exothermic.
Potassium hydroxide soaps are also referred to as soft soaps. For example in an eutectic mixture of sodium hydroxide and potassium hydroxide the dissolution rate is 2 μmh 1 with 1 water but 040 mmh 1 with 85 water 3. NaOH Sodium hydroxide is Soluble in water.
The solubility of a substance fundamentally depends on the physical and chemical properties of the solute and solvent as well as on temperature. In other words they generate heat and give off hydrogen. Mar 02 2009 It is a hygroscopic solid which is highly soluble in water of about 140 g100 ml 20 C and slightly soluble in alcohol.
Differences in Potassium Hydroxide. When dissolved they can each be used as an aqueous solution. For example if sodium is the alkali metal.
Sodium hydroxide potassium hydroxide ammonium hydroxide Most common hydroxides The top two rows explain why so many salt solutions used in the laboratory are sodium or potassium compounds or. KOH is also more soluble in methanol or ethanol than NaOH. While only 100 g of NaOH will dissolve in 100 ml of water 121 g of KOH will dissolve in the same amount of water under the same conditions.
90-95 potassium hydroxide caustic potash is obtained by evaporating potassium hydroxide solution. The residual content of 5-10 H2O is bound as a monohydrate. Jan 13 2020 Because potassium hydroxide and sodium hydroxide are both strong bases they react to water by releasing heat.
The main difference between Potassium hydroxide and Sodium Hydroxide is that Potassium hydroxide has a. When dissolved in water or neutralized with acid it liberates substantial heat which may be sufficient to ignite combustible materials. While it is more common to use sodium hydroxide for soap potassium hydroxide soaps are actually more soluble in water and are better for the environment.
Their chemical similarity lends to a physical similarity as well. Bp 1390 C mp 318 C bp 1320 C mp 360 C Highly soluble in water Highly soluble in water 109 g100 mL Odor. Apr 06 2015 Potassium hydroxide KOH is more soluble in water than Sodium hydroxide NaOH.
Solubility is the property of a solid liquid or gaseous chemical substance called solute to dissolve in a solid liquid or gaseous solvent. Reactivity with water. Potassium hydroxide is less soluble in water.
Jan 23 2018 Potassium Hydroxide. The reaction of potassium hydroxide is less exothermic than the reaction of sodium hydroxide with water. Sodium water sodium hydroxide hydrogen gas 2 Na 2 H 2 O 2 NaOH H 2.
Potassium Hydroxide Assignment Point
Difference Between Potassium Hydroxide And Sodium Hydroxide Definition Properties Applications Similarities And Differences
What Salt Is Formed When Aqueous Potassium Hydroxide React With Ethanoic Acid Quora
 Effect Of Sodium Hydroxide And Potassium Hydroxide In Methanol On The Download Table
Effect Of Sodium Hydroxide And Potassium Hydroxide In Methanol On The Download Table
 Sodium Hydroxide An Overview Sciencedirect Topics
Sodium Hydroxide An Overview Sciencedirect Topics
 Sodium Hydroxide Vs Potassium Hydroxide Reaction With Aluminum Youtube
Sodium Hydroxide Vs Potassium Hydroxide Reaction With Aluminum Youtube
 Sodium Hydroxide Lye Learn All About Lye How To Make Soap Safely
Sodium Hydroxide Lye Learn All About Lye How To Make Soap Safely
 Solved A Student Dissolves 11 1 G Of Potassium Hydroxide Chegg Com
Solved A Student Dissolves 11 1 G Of Potassium Hydroxide Chegg Com
 Potassium Hydroxide An Overview Sciencedirect Topics
Potassium Hydroxide An Overview Sciencedirect Topics
 What S The Difference Between Sodium Hydroxide And Potassium Hydroxide For Soapmaking Rusticwise
What S The Difference Between Sodium Hydroxide And Potassium Hydroxide For Soapmaking Rusticwise
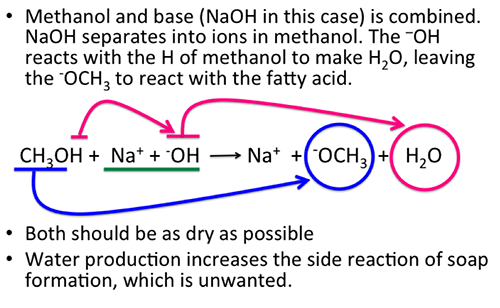 9 2 The Reaction Of Biodiesel Transesterification Egee 439 Alternative Fuels From Biomass Sources
9 2 The Reaction Of Biodiesel Transesterification Egee 439 Alternative Fuels From Biomass Sources
Which Is The Strongest Base Among Naoh Koh Lioh And Csoh Quora
 Difference Between Lye And Caustic Soda Compare The Difference Between Similar Terms
Difference Between Lye And Caustic Soda Compare The Difference Between Similar Terms
 Effect Of Sodium Hydroxide And Potassium Hydroxide In Methanol On The Download Table
Effect Of Sodium Hydroxide And Potassium Hydroxide In Methanol On The Download Table
 Potassium Hydroxide Alchetron The Free Social Encyclopedia
Potassium Hydroxide Alchetron The Free Social Encyclopedia
 How To Balance Hcl Koh Kcl H2o Hydrochloric Acid Potassium Hydroxide Youtube
How To Balance Hcl Koh Kcl H2o Hydrochloric Acid Potassium Hydroxide Youtube
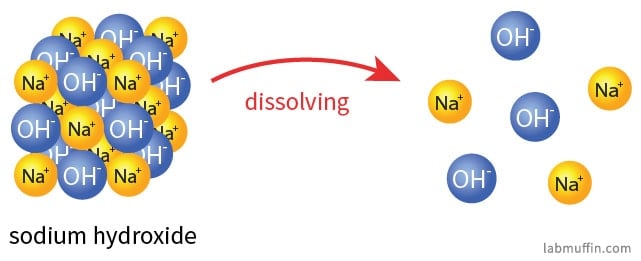 Is Sodium Hydroxide Safe In Beauty Products Lab Muffin Beauty Science
Is Sodium Hydroxide Safe In Beauty Products Lab Muffin Beauty Science
Difference Between Potassium Hydroxide And Sodium Hydroxide Definition Properties Applications Similarities And Differences
Difference Between Alkali And Metal Hydroxide Definition Formation Properties Examples Differences